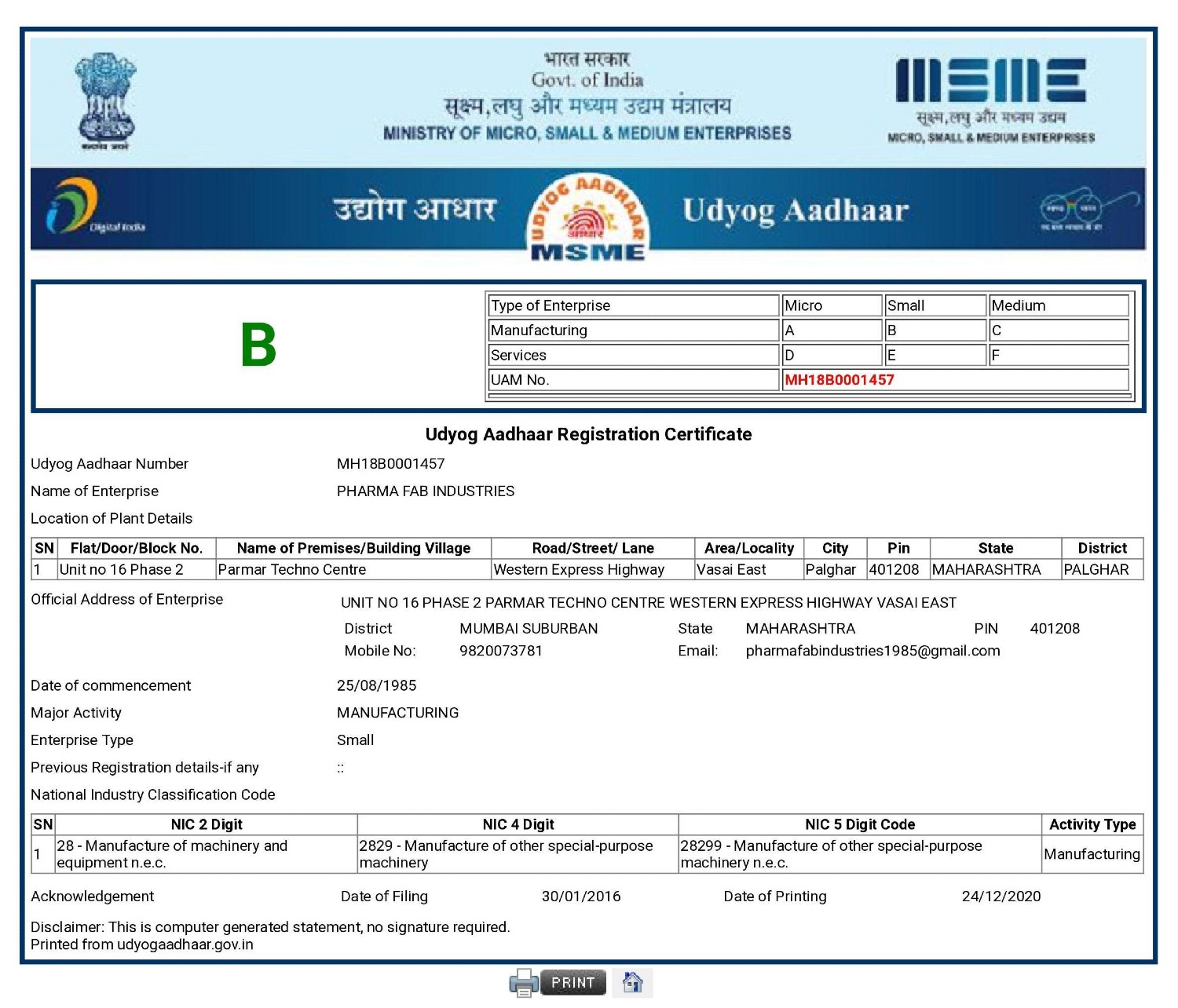
Certification
- Home
- Certification
cGMP CERTIFICATE 2021
CGMP refers to the Current Good Manufacturing Practice regulations enforced by the FDA. CGMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This formal system of controls at a pharmaceutical company, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures, and errors. This assures that drug products meet their quality standards.
ISO 9001 2015 Certificate - 2021
NABCB
NABCB is the acronym for National Accreditation Board for Certification Bodies. NABCB is a constituent of Quality Council of India (QCI). QCI has been established in 1998 through the joint initiatives of the Indian Government and the industries.
The objective of NABCB is to establish and offer accreditation schemes, based on internationally accepted standards, for certification bodies and inspection bodies engaged in providing services of system certification (ISO 9001, ISO 14001 etc.), product certification and inspection.
ISO 9001 2015 Certificate - 2021
UKAS
The United Kingdom Accreditation Service (UKAS) is the national accreditation body for the United Kingdom, appointed by government, to assess organisations that provide certification, testing, inspection and calibration services.
UKAS is appointed as the National Accreditation Body by the Accreditation Regulations 2009 (S.I. No 2009/3155) and Schedule 33 of the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019 (S.I. 2019/696).